Description
| Product Name | Details | CAT.No | Size |
| Microalbumin | Suitable for Hitachi 917 | HIT0471B | R1: 2 x 20ml R2: 1 x 8ml R4: 5 x 1ml |
| Microalbumin | Suitable for use on Olympus System AU600, AU400, AU640, AU2700 | OLY0471C | R1: 2 x 20ml R2: 1 x 8ml |
| Microalbumin | Suitable for CA 180/270/400 Furuno/ Daytona Series Reagents | BXCF417A | R1: 6 x 20ml R2: 3 x 8ml |
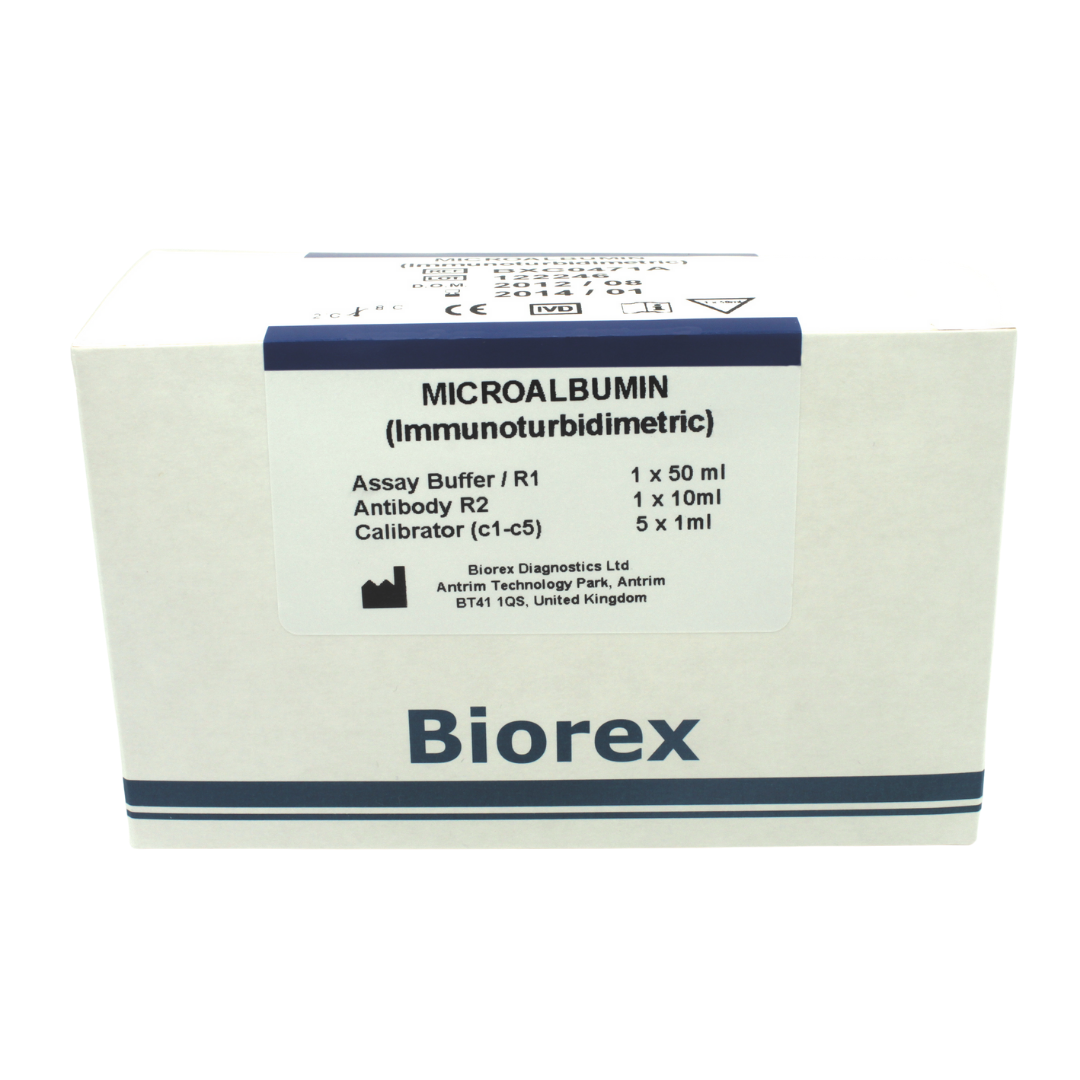
Product Description
The Biorex Diagnostics Microalbumin assay is an in vitro diagnostic test for the quantitative determination of microalbumin in human urine. Microalbumin is an early marker of renal injury and cardiovascular risk.
Key Features
• Quantitative measurement of urinary microalbumin (30–300 mg/day)
• Sensitive marker for early renal dysfunction and diabetic nephropathy
• Immunoturbidimetric or colorimetric method ensures accurate, reliable, and reproducible results
• Supports cardiovascular and renal risk assessment
• Compatible with Hitachi 917, Olympus AU600/400/640, and CA 180/270/400/2700 Furuno/Daytona systems
Benefits
The Biorex Microalbumin Assay provides precise, reproducible, and clinically meaningful results for early detection and monitoring of renal impairment. Its sensitivity and automated compatibility make it essential for clinical laboratories, hospital diagnostics, and research studies.