Description
| Product Name | CAT.No | Size |
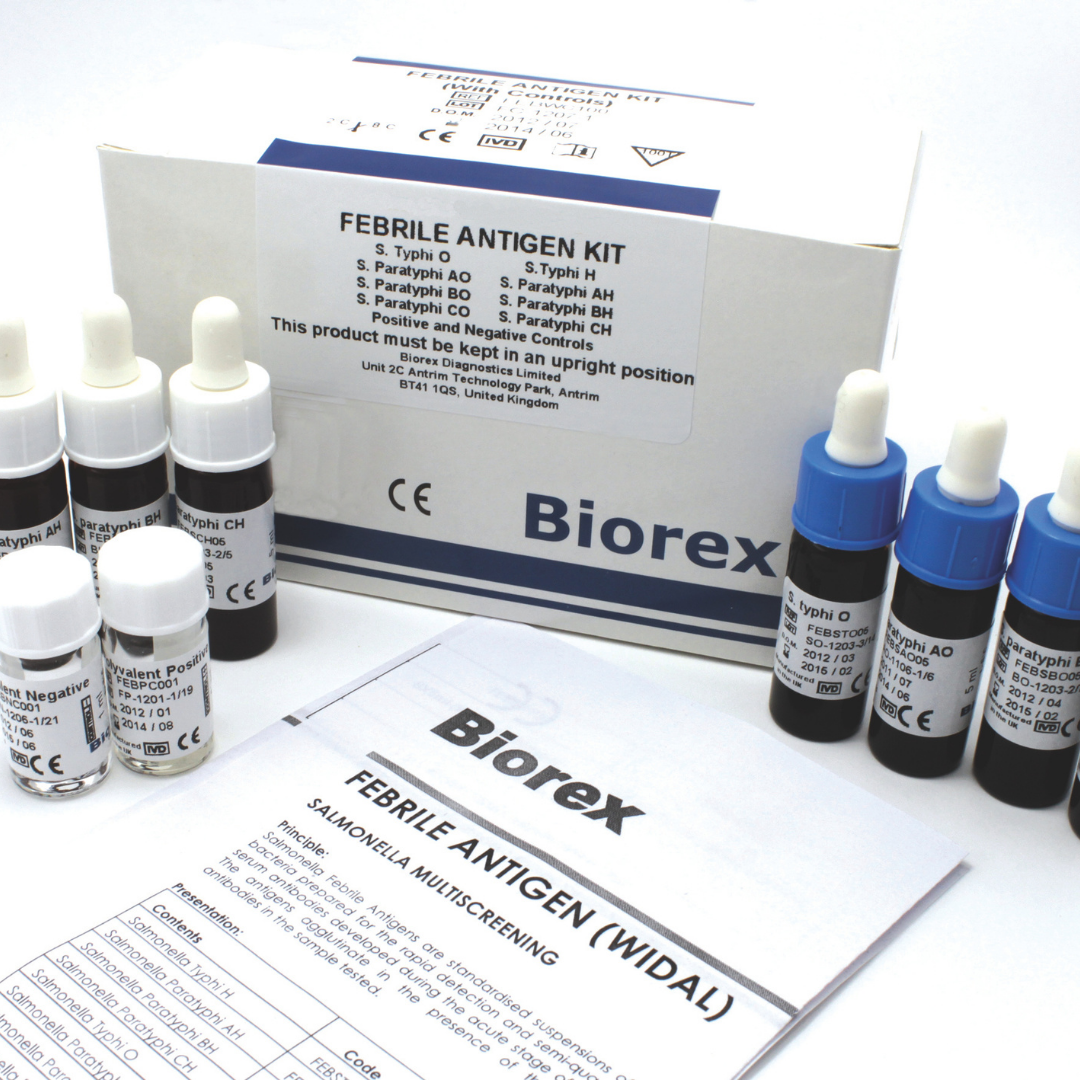
| Febrile Antigen Kit (Widal) Without Controls | FEBNC100 | 8 x 5ml |
| Febrile Antigen Kit (Widal) With Controls | FEBWC100 | 8 x 5ml/2 x 1ml |
| Febrile Negative Controls | FEBNCO01 | 1 x 1ml |
| Febrile Positive Controls | FEBPCO01 | 1 x 1ml |
Product Description
The Biorex Diagnostics Febrile Antigen (Widal) Test is a rapid slide agglutination assay for the qualitative and semi-quantitative detection of antibodies to Salmonella typhi and Salmonella paratyphi A, B, and C in human serum. Designed for dependable serological screening, it supports the diagnosis and monitoring of enteric fever, including typhoid and paratyphoid, in both routine clinical practice and public health surveillance programmes.
This test is based on the well-established Widal agglutination principle, using standardized H and O antigens to identify antibody responses associated with acute or recent infection. Clear and easily interpretable agglutination reactions enable rapid assessment without the need for complex instrumentation, making the assay suitable for laboratories operating in both high-throughput and resource-limited settings.
Key Features
- High sensitivity and specificity – reliable detection of antibodies to Salmonella typhi and Salmonella paratyphi A, B, and C
- Rapid visual results – clear agglutination observed within minutes
- Simple to perform – minimal equipment required; suitable for routine and field use
- Qualitative and semi-quantitative capability – supports screening and antibody titre estimation
- Cost-effective testing solution – ideal for large-scale screening and endemic regions
Benefits
Routine use of the Biorex Diagnostics Febrile Antigen (Widal) Test enables early identification of enteric fever, supporting prompt clinical intervention and effective patient management. The ability to estimate antibody titres provides additional value for monitoring disease progression and response to treatment over time. Its straightforward procedure reduces hands-on time and training requirements, helping laboratories maintain efficient workflows while delivering consistent and dependable results.
The test is particularly valuable for public health surveillance and outbreak control, where rapid screening and high testing volumes are required. By combining speed, reliability, and ease of use, the Biorex Febrile Antigen (Widal) Test supports clinicians, laboratory professionals, and public health teams in improving diagnostic confidence, strengthening disease monitoring programmes, and enhancing patient care outcomes in regions affected by enteric fever.
Contact Us for more information.