Description
| Product Name | CAT.No | Size |
| Salmonella paratyphi A-H | FEBSAH05 | 1 x 5ml |
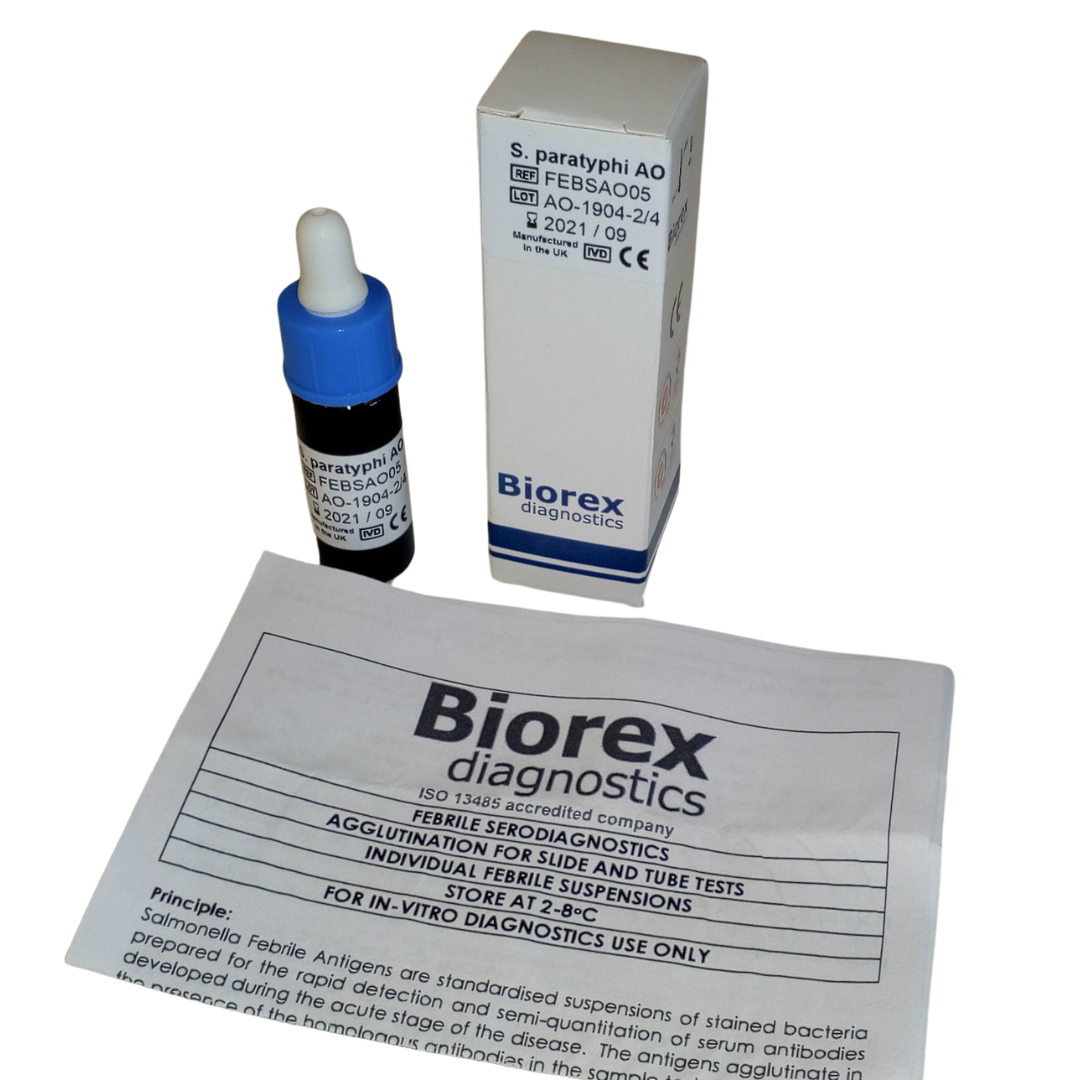
| Salmonella paratyphi A-O | FEBSAO05 | 1 x 5ml |
| almonella paratyphi B-H | FEBSBH05 | 1 x 5ml |
| almonella paratyphi B-O | FEBSBO05 | 1 x 5ml |
| Salmonella paratyphi C-H | FEBSCH05 | 1 x 5ml |
| Salmonella paratyphi C-O | FEBSCO05 | 1 x 5ml |
Product Description
The Biorex Diagnostics Salmonella Paratyphi Febrile Antigen (Widal) Test is a rapid slide agglutination assay designed for the qualitative and semi-quantitative detection of antibodies against Salmonella paratyphi A, B, and C in human serum. This test provides a reliable and efficient method for the diagnosis of paratyphoid fever, making it a valuable tool for clinical laboratories, hospitals, diagnostic centres, and field screening programs. Its simple and rapid procedure ensures timely results, supporting effective patient management and public health interventions in both routine and outbreak scenarios.
Key Features
- High sensitivity and specificity – accurately detects antibodies to Salmonella paratyphi A, B, and C
- Rapid visual results – clear agglutination allows quick interpretation within minutes
- Simple workflow – requires minimal laboratory equipment and training, suitable for both field and hospital use
- Multi-antigen detection – supports identification of specific paratyphoid strains for definitive diagnosis
- Cost-effective – ideal for high-throughput laboratory testing and large-scale epidemiological studies
Benefits
Routine use of the Biorex Salmonella Paratyphi Febrile Antigen Test enables early identification of paratyphoid fever, facilitating timely clinical intervention and improved patient outcomes. Its semi-quantitative capability allows monitoring of antibody titres, providing insights into disease progression and treatment response. The rapid slide agglutination method streamlines laboratory workflow, reduces turnaround time, and supports efficient testing in high-volume diagnostic settings.
In addition to patient care, this test is highly beneficial for public health surveillance, assisting healthcare authorities and epidemiologists in tracking the spread of paratyphoid fever and implementing appropriate control measures. Its ready-to-use format minimizes preparation errors, ensures reproducible results, and supports consistent diagnostic confidence across multiple samples and laboratory environments.
By incorporating the Biorex Paratyphi Widal Test into standard diagnostic protocols, laboratories can maintain high standards in enteric fever detection, enhance clinical decision-making, and contribute to effective disease monitoring and prevention. The combination of speed, accuracy, and ease of use makes this test a reliable solution for hospitals, diagnostic centres, and field-based epidemiological programs.
Contact Us for more information.